***Important *** Classification, Labelling and Packaging of Anticoagulant Rodenticide changes effective 1st March 2018
Implementation of the 9th ATP Regulation to Anticoagulant Rodenticides
CLP Reg. No. 1272/2008:
Impact on rodenticide labels and legislation effective from 1st March 2018.
We have recently communicated with our customers about the Classification, Labelling and Packaging (CLP) changes affecting anticoagulant rodenticides for amateur use (active substance below 30 parts per million (PPM)). Another major change within this legislation is that any anticoagulant rodenticide that contains above 29 ppm of active substance (i.e. for professional use) will now require a reprotoxicity pictogram and hazard statement.
From 1st March 2018 all anticoagulant rodenticides with an active concentration of above 29ppm must be CLP compliant and labelled accordingly with the pictogram and hazard statement shown below: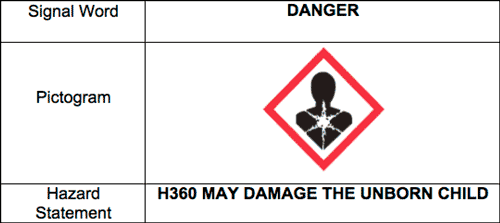
The impact on this new classification is:
- Products containing 50ppm of the anticoagulant active ingredient authorised for use in the Professional market will need to be relabelled to become compliant with the CLP regulations.
- The minimum pack size for professional use rodenticides is set at 3KG.
The responsibility for ensuring that products are correctly labelled falls on all distributors in the supply chain making these products available into the market. It is permitted for these products to be over-labelled with the updated classification before they are made available for sale onto the market from 1st March 2018.
Additionally, the updates to the product classification mean that both the product labels and the Manufacturers Safety Data Sheets (MSDS) of these products will change, thereby changing the risk assessments for the use of the product. Should you need a copy of the product label or MSDS please refer to our website. Alternatively, please call the sales team on 01384 404242.
As well as the CLP regulation, anticoagulant rodenticides are going through a Re-authorisation process which should be completed in the next few months. As a result of this, further label changes are very likely in terms of text and usage. A further update on this will follow in due course.


